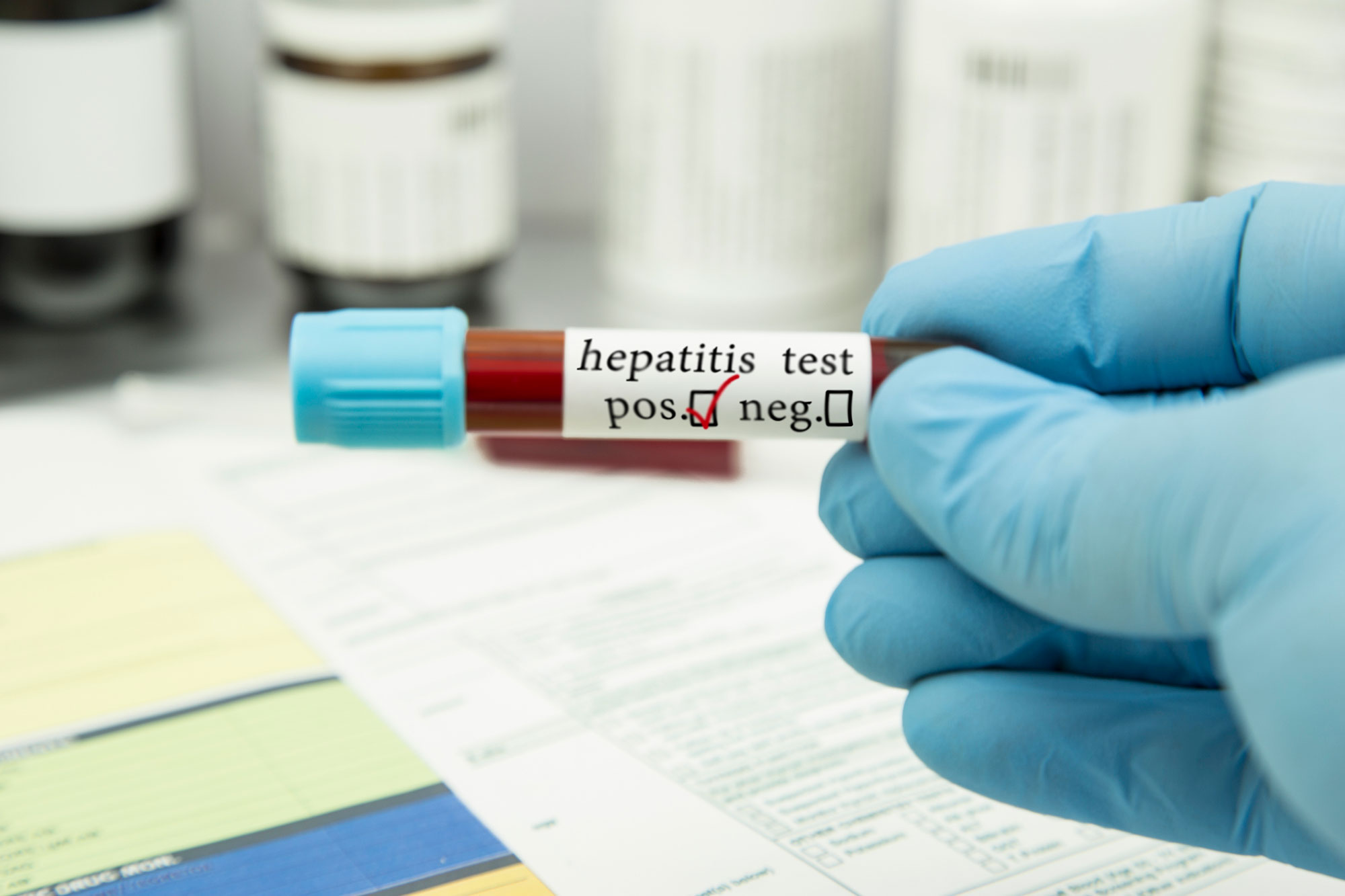
HEPATITIS, a health condition that attacks vital human organs, appears more dreadful to human survival, given its wide range of transmission modes and the attendant chances of survival.
However, the Director-General of the Ghana Health Service (GHS), Dr Patrick Kuma-Aboagye, says hepatitis is not a death sentence, and that early detection and treatment are critical to preventing the disease from advancing to more severe stages, such as chronic hepatitis or cirrhosis.
To him, “Early detection of viral hepatitis is essential for timely treatment, preventing disease progression and transmission, reducing complications and improving overall public health outcomes. Regular screenings, especially for high-risk populations, play a crucial role in identifying infections early and ensuring appropriate medical care.”
He cautioned that the mode of transmitting viral hepatitis – through body fluids – made everybody vulnerable to the infection, which could be very deadly but manageable and curable in some instances.
Common modes of transmission include unprotected sexual contact, sharing of contaminated needles or syringes, and from mother to child during childbirth or breastfeeding.
Other strains could also happen if contaminated food or water are consumed, or through direct contact with an infected person’s faeces.
He revealed that the low-hanging fruit for everybody in preventing and keeping an infection under control, was screening.
“Hepatitis can sneakily infiltrate the body and cause damage before any symptoms appear.
“Early detection through screening allows for timely intervention, preventing the virus from wreaking havoc on the liver and reducing the risk of severe complications. Hepatitis viruses can cause inflammation and damage to the liver over time,” he said. “Some individuals infected with hepatitis viruses may be asymptomatic but can still transmit the virus to others.” The Director General called on all citizens to get screened immediately.
He said that would enable the country to realise its target to diagnose 90 per cent of Hepatitis B and C cases and put at least 80 per cent on treatment to help nip the growing burden in the bud.
The Director-General said some hepatitis infections were curable while others were only suppressible to enable an infected person to live a normal life.
That, Dr Kuma-Aboagye said, meant that the condition was not a death sentence if picked early and put on treatment.
Viral Hepatitis
Hepatitis is a medical term used to describe inflammation of the liver. It can be caused by various factors, but the most common cause is viral infections.
There are five main types of viral hepatitis, labelled as hepatitis A, B, C, D, and E. Each type is caused by a different virus and has distinct characteristics.
But their modes of spread are pretty similar – through contact with the fluid or blood of an infected person.
Some forms of viral hepatitis, especially hepatitis B and C, can be transmitted from person to person through various means, such as unprotected sexual contact, sharing needles, or from mother to child during childbirth.
Symptoms of viral hepatitis could vary depending on the type of virus and the stage of the infection. Some common symptoms include fatigue, jaundice, loss of appetite, nausea and vomiting, among others.
It is, therefore, important to note that not everyone infected with viral hepatitis will exhibit symptoms. Some people may experience a mild illness or have no symptoms at all, especially during the is early stages of infection.
Depending on the specific virus. (Hepatitis A, B, C, D, or E), antiviral medications, vaccines and other interventions could help manage the infection, prevent complications and improve the chances of recovery.
Health experts say there is currently no effective vaccine against hepatitis C, but an early diagnosis can prevent the health problems that may result from infections and prevent transmission of the virus.
Also, there are effective tools available to prevent viral hepatitis infection, which include hepatitis B vaccination, surveillance, education, screening and treatment.
Preventing hepatitis involves practicing good hygiene, getting vaccinated (where available) for hepatitis A and B, practicing responsible sex, avoiding sharing needles or personal items that may come into contact with blood or body fluids and following safe medical procedures.
To a hepatologist, Dr Adwoa Agyei- Nkansah, Hepatitis C was curable. However, the cost of treatment and testing was expensive, which placed a lot of poor patients at a disadvantage.
She revealed that several discussions were ongoing with some diagnostic centres in the country to subsidise the diagnosis of patients in an attempt to encourage more persons to check their status, seek medical care and eventually eliminate Hepatitis from the country.
Checks by the Daily Graphic showed that the pre-test examinations cost between GH¢2,000 and GH¢3,000 while treatment costs close to GH¢7,000, an amount which was beyond the reach of many people in the country.
Testing Hesitancy and National Burden
Recently at the launch of World Hepatitis Day, the Programme Manager, National Viral Hepatitis Control Programme, Ghana Health Service (GHS), Dr. Atsu Godwin Seake-Kwawu, said although the country was experiencing a growing burden of hepatitis infections and mortalities, it had very low rates of diagnosis, treatment and awareness.
He revealed that there were 1.5 million people with Hepatitis B and C in the country, with over 3,000 deaths every year from liver cancer and cirrhosis.
However, due to testing hesitancy, only 10 per cent of people with chronic Hepatitis B (HBV) were diagnosed, of which only 22 per cent received treatment.
For Hepatitis C, only 21 per cent of people with the infection are diagnosed, with 62 per cent of those diagnosed receiving treatment to cure them.
Although four out of every 100 people sampled have the condition, the national response is said to be plagued with the high cost of pre-treatment examinations and treatment and limited access to treatment.
Dr. Seake-Kwawu encouraged all, particularly pregnant women, to get tested for the disease for early treatment to prevent those with a high viral load from sharing it with their unborn babies.
Elimination
Dr. Seake-Kwawu said hepatitis was a global condition and that the World Health Organization (WHO) was working towards its elimination by 2030.
He urged Ghanaians to regularly test for hepatitis to know their status for early treatment. He added that the GHS was increasing its activities towards the elimination of viral hepatitis in the country.
He said the service had started the implementation of a project to screen pregnant women for the condition and vaccinate them after delivery to curtail the spread of the virus.
Dr Seake-Kwawu said it was important to increase prevention, testing, access to treatment and chronic care, as the cost of medication for treatment was expensive.
Writer’s email: doreen.andoh@graphic.com.gh
Published by: The Ghanaian Times Newspaper, 17th August,2023, page 11
Author: Doreen Andoh [...]
Read more...
A NEW invasive mosquito species said to be deadlier than the traditional anopheles mosquitoes, which transmits malaria, has been identified in the country.
“Anopheles stephensi,” first detected on the African continent in 2019, is known to transmit two malaria parasites; Plasmodium falciparum and Plasmodium vivax, which pose the greatest risk of severe illness and death from malaria.
According to the Ghana Health Service (GHS), the strain was confirmed in March this year, from samples taken in Tuba and Dansoman, all suburbs in the Greater Accra Region, thereby cautioning the public to take immediate steps to protect themselves from mosquito bites.
“Anopheles Stephensi, can practically breed in almost all sources of water, which is not the traditional breeding sites of the common anopheles species.
It survives in extremely high temperatures during the dry season when malaria transmission usually declines.
It is known to be very invasive, spread fast and adaptive to a myriad of climatic conditions and shown to be resistant to multiple insecticide classes in many locations, posing challenges in its control, a statement issued by the GHS to all Regional Health Directors and sighted by the Ghanaian Times, noted.
In order to mitigate spread of the vector and sustain gains made in the malaria fight, the statement, authorised by the Director-General (D-G), Dr Patrick Kuma-Aboagye, said a national taskforce has been established to advice on strategies to minimise its impact across the country.
He charged Regional and District Health Directors to implement a number of measures including the removal of water collection points in and around homes and communities to minimize breeding sites. The D-G further urged the general public to use insecticide treated nets to protect themselves against indoor mosquito bites, use repellents and wear clothes which protect them against mosquito bites when outside the home.
According to projections by the World Health Organization (WHO), A. stephensi could put an additional 126 million people in Africa at risk of malaria if spread of the mosquito vector is unchecked.
So far, the mosquito specie has been detected in countries including Ethiopia Sudan, Somalia, and Nigeria in sub-Saharan Africa.
Meanwhile, Ghana continues to make breakthrough in the fight against malaria as it has become the first African country to approve a new malaria vaccine from the Oxford University, UK. The vaccine, known as R21/ Matrix-M, has been approved for use in children aged between five and 36 months who are at the highest risk of death from the disease.
It becomes the second malaria vaccine to be approved under the Malaria Vaccine Implementation Programme (MVIP) after MOS-QUIRIX, RIS,S malaria vaccine.
Published by: The Ghanaian Times Newspaper, 14th April,2023, page 11
Author: Times Reporter. [...]
Read more...
The Executive Director of Hope for Future Generations (HFFG), a non-governmental organisation, Cecilia Senoo, has stated that consistent education and sensitisation is the only way to achieve a generation free from HIV infections.
She said people needed to understand that the disease was still around and for that reason, there was the need for them to consistently protect themselves and their partners.
“We cannot really achieve an HIV-free generation if we don’t protect, educate and take good care of ourselves so we need to create awareness of HIV prevention and one way to do that is with condoms”, she said.
Campaign
Mrs. Senoo said that on the sidelines of a sensitisation event held in at the Kaneshie Market Lorry Park in Accra last Thursday.
It was in collaboration with the United Nations Population Fund (UNFPA), formerly the United Nations Fund for Population activities and the Ghana AIDS Commission.
Dubbed, “Condom activation campaign”, a booth accompanied by commentary via a public address (PA) system was set up to educate people.
Staff of both parties went round to share condoms for the market people, while educating them on how and why they should use them.
Similarly, they distributed pamphlets that carried key messages on the prevention of HIV, as well as ways to prevent and also educated people on the disease.
There was also an onsite HIV testing for people who wanted to know their status.
Different method
Mrs. Senoo also disclosed that the event was being used to prepare the whole country towards the World Aids Day, which is on December 1.
“So, we are creating awareness because of late, Ghana is recording very high HIV prevalence especially among young people and that means that we are having unprotected sex, which is the most common way to contract the infection”, she explained.
The executive director also noted that the organisation was partnering with the market women as a means to reach people at the grassroots to ensure the message was heard by all.
“We will be using testimonies of people who have used it to protect themselves while having partners who are HIV positive”, she said.
Significance
The Doctor in-charge of the Kaneshie Polyclinic, Dr. Stella Gyamfi, said the exercise was important because there had been a surge in the number of new infections, particularly among the youth.
“This exercise is very relevant because this year alone between January and June, there have been over 23,000 new HIV infections in Ghana.
“Greater Accra alone over 4,000 new HIV infections among them, the youth are more, she noted.
Dr. Gyamfi, who doubles as the Okaikoi South Health Director, also said it was particularly important for the youth because they had proven to be the most vulnerable to infections due to their high sexual activeness, which was a characteristic of the youth.
“People should be educated more on the benefits of condoms with emphasis on its proper use being demonstrated to increase the uptake of condoms”, she added.
Published by: Daily Graphic, 29th November,2022
Author: Dickson Worlanyo Dotse [...]
Read more...
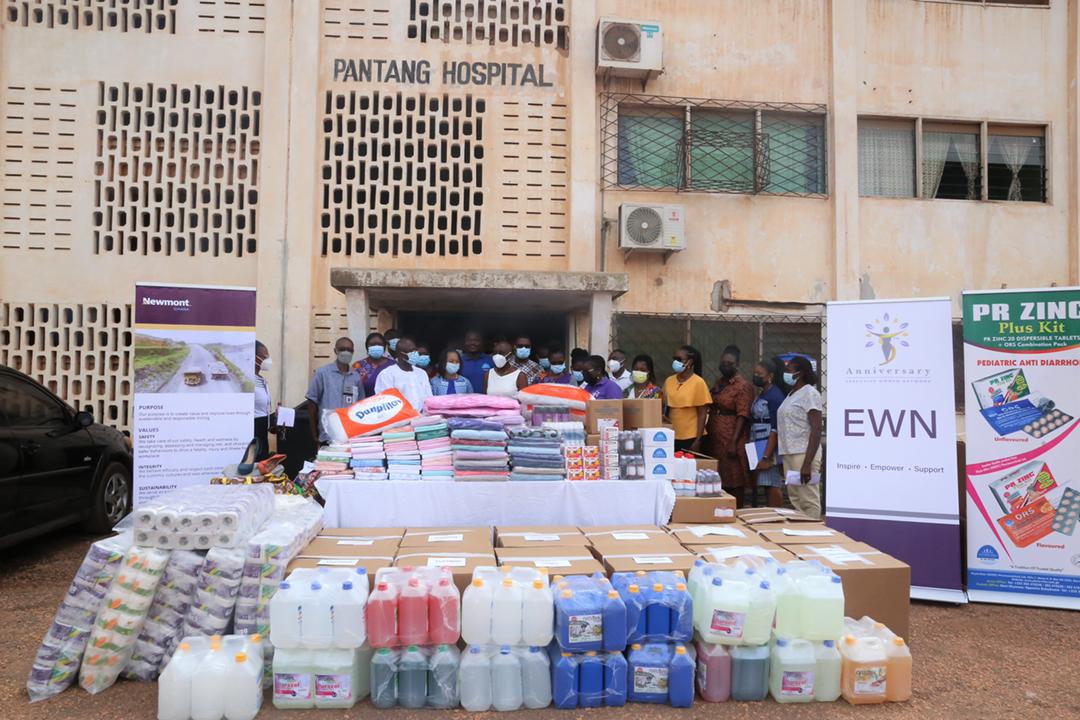
The Executive Women Network (EWN) in collaboration with Newmont Ghana and Phyto-Riker (GIHOC) Pharmaceuticals Ltd has donated clothing, bedding and medical essentials to support mental healthcare delivery in the Pantang Hospital, Accra.
The items donated include; 93 set of Bedsheets and pillow cases, 50 pillows, 68 towels, 700 rolls of toilet paper, 20 gallons of Cleaning Solution, 20 gallons of Disinfectant, 20 gallons of Hand Soap, 10 gallons of Rubbing Alcohol, 120 Hand Sanitizer Spray bottles, 4 cartons of pure hand sanitizer and rubbing alcohol, used clothing and household items. The medical essentials donated by Phyto-Riker Pharmaceuticals were 10 packs of Diazepam, 4 cartons of Ritonic Syrup, 4 of cartons PR Zinc Plus kits, and 4 cartons of Fenamol.
Speaking on the donation, the Chairperson of the Executive Committee of EWN, Mrs. Eunice Biritwum said the donation forms part of EWN’s activities to mark its fifth anniversary.
“This year, we are celebrating our fifth anniversary under the theme: ‘Celebrating Women of Impact’ with a tagline, ‘We See You. Be Bold’. The essence of this theme is to take stock of our five-year journey, acknowledge and recognize the contributions of our amazing EWN women, and to also give back to the community”.
“We do recognize the importance of giving back to our community. We do know that giving promotes a sense of cooperation and strengthens our ties with others, and that is why we were honored and happy to come together with Newmont Ghana and Phyto-Riker towards the donation at Pantang”, she added.
Commenting on the partnership, Regional Vice President, Human Resources at Newmont Ghana, Awo A. Quaison-Sackey expressed her gratitude to EWN for partnering Newmont Ghana for a worthy cause such as this.
“Our new normal-living with COVID-19, has further increased the stress burden in our world today. With people losing loved ones and colleagues, being forced out of their jobs and reducing physical social interaction, we must pay more attention to mental health than we have ever done else the consequences will be very dire. Today’s donation is another way of reducing the burden on vulnerable patients of this facility by providing clothing, bedding and medical essentials. To the Executive Women Network, for their sense of compassion and kindness, I say ‘thank you’ for partnering with us on this worthy cause. To Phyto-Riker, thanks for partnering with the Executive Women Network”.
On her part, Chief Executive Officer for Phyto-Riker (GIHOC) Pharmaceuticals Ltd, Theresa Yamson said her outfit is elated to support with specific pharmaceutical products.
“On behalf of the management and staff of Phyto-Riker, we are delighted to be here this morning to support the medical personnel and patients of the hospital with specific pharmaceutical products that have been manufactured here in Ghana to improve the health and mental well-being of our cherished clients. We are even more excited to be doing this in partnership with Newmont Ghana, and the Executive Women Network of which I am also a proud member”.
EWN is a non-profit organization made up of over 170 phenomenal women, comprising over 120 corporate women in senior management and executive positions of private organizations, and over 50 women entrepreneurs of well-established businesses in Ghana.
[...]
Read more...
… things to note.
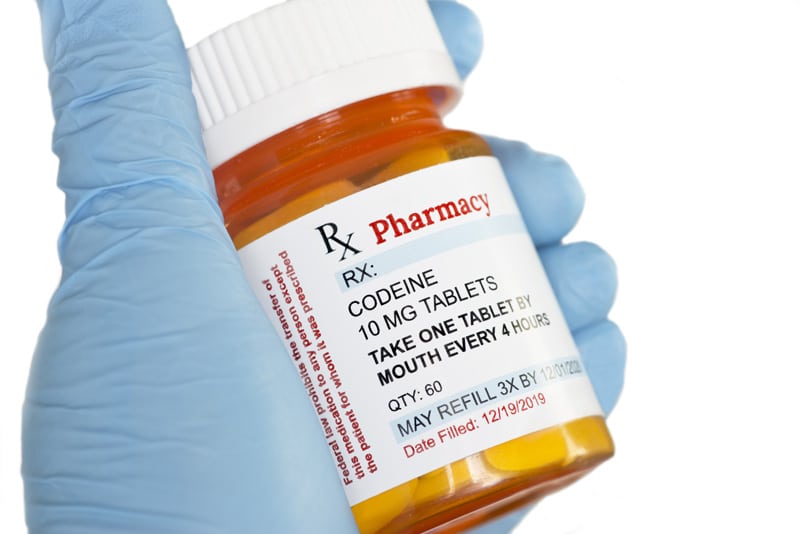
In light of the reported widespread abuse of tramadol in the country, codeine use seems to be on the rise. What we need to remember is that it is an opioid and also liable to abuse. It has its place in the management of pain.
The WHO analgesic ladder was a strategy proposed by the World Health Organization , in 1986, to provide adequate pain relief for cancer patients [Anekar et al. Statpearls.http:// www.ncbi.nlm.nih.gov/books/NBK554435/].
The analgesic ladder was part of a vast health program termed the WHO Cancer Pain and Palliative Care Program aimed at improving strategies for cancer pain management through educational campaigns, the creation of shared strategies, and the development of a global network of support. This analgesic path, developed following the recommendations of an international group of experts, has undergone several modifications over the years and is currently applied for managing cancer pain, and acute and chronic non-cancer painful conditions due to a broader spectrum of diseases such as degenerative disorders, musculosketal disease, neuropathic pain disorders and other types of chronic pain.
The original ladder mainly consisted of three steps:
First step. Mild pain: non-opioid analgesics such as nonsteroidal anti-inflammatory drugs or acetaminophen with or without non-opioid analgesics, and with or without adjuvants.
Second Step. Moderate Pain: weak opioids (hydrocodone, codeine, tramadol) with or without non-opioid analgesics, and with or without adjuvants.
Third step. Severe and persistent pain: potent opioids with or without non-opioid analgesics, and with or without adjuvants.
The term adjuvant refers to a set of drugs belonging to different classes. Although their administration is typically for indications other than pain treatment, these medications can be of particular help in various painful conditions. Adjuvant, also called co-analgesics, include antidepressants including tricyclic antidepressants such as amitriptyline and nortriptyline, serotonin-norepinephrine re-uptake inhibitors such as duloxetine and venlafaxine, anticonvulsants like gabapentin and pregabalin, topical anesthetics , topical therapies , corticosteroids, bisphosphonates, and cannabinoids.
The key concept of the ladder is that it is essential to have adequate knowledge about pain, to assess it degree in patient through proper evaluation, and to prescribe appropriate medications. As many patients will receive opioids eventually, it is essential to balance the optimum dosage with the side effect of the drug. Opioid rotation can be adopted to improve analgesia and reduce side effects. Patient should receive education about the uses and side effects of drugs to avoid misuse or abuse without compromising their beneficial aspects.
The real limitation of the original scale was the impossibility of integrating non-pharmacological treatment into the therapy path. As a consequence, a fourth step was added to the ladder. It includes numerous non-pharmacological procedures that are robust recommendations for treating persistent pains, even in combination with the use of strong opioids or other medications. This group encompasses interventional and minimally invasive procedures such as epidural analgesia, intrathecal administration of analgesic and local anesthetic drugs with or without pumps, neurosurgical procedures (e.g., lumber percutaneous adhesiolysis, cordotomy), neuromodulation strategies (e.g., brain stimulators, spinal cord stimulation), nerve blocks, ablative procedures (e.g., alcoholization, radiofrequency, microwave, cryoablation ablations; laser induced thermotherapy, irreversible electroporation, electrochemotherapy), cementoplasty and palliation radiotherapy.
The updated WHO analgesic ladder focuses on the quality of life and adopts a bidirectional approach, extending the strategy to treat acute pain as well. For acute pain, the strongest analgesic (for that intensity of pain) is the initial therapy and later toned down, whereas, for chronic pain, employing a step-wise approach from bottom to top. The International Association for the study of Pain (IASP) adopts a therapeutic approach more focused on the type of pain (i.e., mechanism) and on the mechanism of action of the drugs used to treat it. Therefore, in the case of chronic nociceptive pain on an inflammatory basis, it would be more appropriate to use steroids or NSAIDs. On the other hand, low-inflammatory nociceptive pain should receive treatment with opioids and non-opioid analgesics. Neuropathic pain may require antidepressants or anti-convulsant, and specific drugs in certain rheumatologic clinical conditions (e.g, colchicine to treat gout)
Everyone is likely to experience pain at least once in a life, either acute or chronic. Indeed, pain is the most common reason patients see their doctors or access to an Emergency Department (ED) (Franceschi et al. Safety and efficacy of the combination Acetaminophen-codeine in the treatment of pain of different origin. European Review for Medical and Pharmacological Sciences.2013;17:2129-2135).
Pain is a very complex phenomenon that simultaneously involves different spheres, including sensorial, emotional and spiritual. The complexity could explain why so many different molecules may be effective in the treatment of different kinds of pain. The World Health Organization (WHO) promotes and supports optimal pain relief as a fundamental human right, developed an analgesic ladder to guide healthcare providers in pain management.
There are different methods to assess pain treatment: most of the studies published on pain treatment evaluate the efficacy of a specific analgesic by means of the visual analogue scale (VAS). This scale can be used to easily evaluate pain severity and relief, and the effect of different drugs on pain. Acetaminophen/Codeine combination represents the standard medication in the second step of the WHO analgesic ladder and is the most commonly used opioid analgesic for a variety of pain conditions. The combination of two different drugs acting centrally and peripherally, or potentiate both efficacy and tolerability. Codeine is a selective μ opioid receptor agonist that is converted to the more active compound morphine after a hepatic O-demethylation. However, this conversation is not equal in all patients due to generic polymorphism among different ethnic groups and could reflect in varying severity of adverse effects. Adverse effects of opioids include nausea, constipation, abdominal pain, respiratory depression, urinary retention, sedation, pruritus and dependence. Acetaminophen/Codeine combination is recommended for pain not controlled by Acetaminophen alone.
The Acetaminophen/Codeine combination is effective in the treatment of mild to moderate pain in all the setting analyzed by different studies published in general literature.
Codeine (3-methylmorphine) is a mild opioid with analgesic and antitussive effect. Its analgesic activity is mostly due to the conversion to morphine by the cytochrome P450 enzyme CY-P2D6. In the context of analgesic action, Codeine can be considered as a prodrug. Only around 10% of codeine is converted to morphine. It has been found that a combination of different analgesic drugs at fixed doses, rather than their single use, leads to a faster and better acute pain relief. The main purpose of such combination is the synergistic analgesic effect due to the multimodal approach to pain processing, alongside the better safety profile with no increase of the adverse effect incidence due to the initial lower doses of individual analgesics .The combination of non-opioid analgesic/antipyretic drugs, like paracetamol, ibuprofen, or acetylsalicylic acid with codeine is rational since these substances have different mechanisms of action on pain with greater analgesic potential being achieved, without reaching drugs individual toxic limits. It is worth mentioning that doses of codeine in combinations with other drugs vary significantly, from 8 up 60mg.
Culled from the features page: Your Health – Ghana Times Newspaper, 23rd March 2022.
Article by Dr. Edward O. Amporful
Chief Pharmacist, Cocoa Clinic [...]
Read more...
“Edward, I take Atacand 16mg for hypertension. I have been given another one today. I was told it is Candesartan 16mg and it is the same as Atacand 16mg”.
Atacand is innovator medicine. After the expiration of the innovator company’s patent, other companies can produce provided they meet certain conditions. These are referred to as generics. A company may also decide to produce this off-patent medicine under a unique name – that is a branded generic.
Generic medicines are those where the original patent has expired and which may now be produced by manufactures other than the innovator (patent-holding) company . The World-Wide Health Organization (WHO) defines generic medicine as a pharmaceutical product which is usually intended to be interchangeable with an innovator product, is manufactured without license from the innovator company and is marketed after the expiry date of the patent or other exclusive rights. One of the main principles underpinning the safe and effective use of generic medicines is the concept of bioequivalence (Dunne et al. A review of the differences and similarities between generic drugs and other originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC pharmacology and toxicology 2013, 14:1).
Two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and their bio availabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same route of administration in the same molar dose are similar to such a degree that their effects with respect to both efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same amount of the same active substances in the same dosage form, for the same route of administration and meeting the same or comparable standards.
The main purpose of generic drug development is to reduce the price of marketed drugs (Tacca et al. Lack of pharmacokinetic bioequivalence between generic and branded amoxicillin formulations. A post marketing clinical study on healthy volunteers. British journal of clinical pharmacology 68:1 / 34-42). Accordingly, the regulatory authorities of several countries, including the food and drugs administration, the Europe Agency for the evaluation of medicinal products (EMEA) and the World Health Organization (WHO), have issued guidelines illustrating the terms and conditions under which generic drug products can be reorganized as therapeutically equivalent to their brand name counterparts. The chemical composition of generic formulations may differ from their respective brand products. Indeed, the use of different excipients is commonly allowed by international guidelines under specific terms and conditions. As regards the active ingredients, these molecules can be present in generic formulations as different salts or polymorphic species of the leader compound. In particular, the EMEA guidelines designates as ‘pharmaceutical alternative’ a medicinal product that contains a different chemical from (i.e., salt, ester, etc.) of the active ingredient present in the brand leader.
The MSD Manual Merck Manual (August 2020) has reviews on this issue for customers. I have used portions for this piece. Although 16mg of a brand name chemical is identified to 16mg of the same generic chemical (Candesartan), a 16mg generic pill containing that chemical may or may not have the same effect in the body as a 16mg brand-name pill. A first-time switch like this in a chronic disease patient will require monitoring and counselling by the pharmacist.
Ingredients used in a particular product formulation affects how it is absorbed into the blood stream. Inactive ingredients such as coatings, stabilizers, fillers, binders, flavoring, diluents and others are necessary to turn a chemical to a usable drug product. These ingredients maybe used to provide bulk so that a tablet is large enough to handle, keep a tablet from crumbling between the time it is manufactured and the time it is used, help a tablet dissolve in the stomach or intestine, or provide a pleasant taste and color. Inactive ingredients are usually harmless substances that do not affect the body. However, because inactive ingredients can cause unusual and sometimes severe allergic reactions in a few people, one version, or brand, of a medicine may be preferable to another. For example, chemicals called bisulfites (such as sodium metabisulfite), which are used as preservative in many products, cause asthmatic allergic reactions (wheezing, shortness of breath, chest tightness) and many more. Consequently, drug product containing bisulfites are prominently labeled as such.
Manufacturing must conduct studies to determine whether their version is bioequivalent to the original medicine that is, that the generic version releases its active ingredients (the medicine) in the blood stream at virtually the same speed and in virtually the same amount as the original medicine. Because the active ingredient in generic has already been shown in testing of the brand name to be safe and effective, bioequivalence studies only have to show that the generic version produces virtually the same level of drug in the blood overtime and thus require relatively a small number (24 to 36) of healthy volunteers.
Although people generally think of oral dosage forms, such as tablets, capsules and liquids when they think about generic prescription medicines, generic versions of other drug dosage forms such as injections, patches, inhalers and others, must also meet a bioequivalence standard.
Theoretically any generic medicine that is bioequivalent to its brand name counterparts maybe interchanged with it. For medicines that are off patent, the generic medicine may be the only form available. In cases where small differences in the number of medicines in the bloodstream can make a very large differences in the medicine’s effectiveness, generic medicines are often not substituted for brand name medicines, although bioequivalent generic products are available. The alternative is to stick to one particular brand in such circumstances. Example, warfarin, phenytoin. Medicines that must be given in very precise amounts are less likely to be interchangeable, because the differences between an effective dose and harmful dose (the margin of safety) or ineffective dose is small. Example, Digoxin.
Culled from the features page: Your Health – Ghana Times Newspaper, 27th April 2022.
Article by Dr. Edward O. Amporful
Chief Pharmacist, Cocoa Clinic
[...]
Read more...
Phyto-Riker (GIHOC) Pharmaceuticals Limited (PRG), formerly Ghana Industrial Holding Corporation Pharmaceuticals (GIHOC), turns 60 years this year and the company will celebrate its Diamond Jubilee in recognition of the feat it has chalked over the years.
The company was birthed in 1962, five years after Ghana gained independence, as part of a national drive to set up a state-owned pharmaceutical company to produce outstanding quality medications.
Through the government’s privatization of state-owned enterprises, GIHOC was divested and became Phyto-Riker (GIHOC) Pharmaceuticals Limited PRG, an American company.
Through a consortium of locally pooled investors, it has reverted back into wholly Ghanaian ownership and management!
PRG’s mission is to offer high-quality pharmaceutical products and related services, at an affordable cost, to meet the needs of the population of Ghana, West Africa, and beyond.
Today, due to the dynamism of PRG’s board and leadership, the company is uniquely positioned in the sub-Saharan African market to manufacture brands and products specifically tailored to the African marketplace.
Vibrant brands
Phyto-Riker (GIHOC) Pharmaceuticals Limited (PRG) became the first ISO 9001-2015-certified pharmaceutical manufacturing company in West Africa, leveraging on its West African network and increasing Ghanaian market share to chart a path critical to leapfrogging into the enormous promising future ahead.
The company has over the years registered over 70 pharmaceutical products with the Food and Drugs Authority (FDA) in compliance with the provisions of the Public Health Act 2012 (851).
The product portfolio ranges from anti-infectives, anti-malarial, and analgesics including pediatric medications.
Some of the leading brands of PRG include ZUBES®️ Cough Range, DRASTIN®️, CETAPOL®️, CIPROMAX®️, FLEMEX®️, DIPHEX®️, RITONIC®️, RIKER-ON®️, PR ZINC®️ Tablets, and PR ORS®️, among others.
Operational countries
Quite a good number of the company’s products are registered across different West African Anglophone and Francophone countries such as Ghana, Liberia, Senegal, Sierra Leone, Mali, The Gambia, Niger, Nigeria, Benin, Togo, Cote D’Ivoire, and Burkina Faso.
With approximately 200 dedicated employees working in the company factory in Accra and Kumasi, PRG continues to make an enormous contribution to the socio-economic development of Ghana.
To drive continuous value for all stakeholders with utmost support from the board, PRG is led by a top-notch management team covering key units of Administration, Finance, Sales & Marketing, Product Development, Manufacturing, Quality Assurance / Control, Supply Chain, Human Resources Management and Engineering.
Innovation capabilities
Building on a product portfolio of both branded and generic products, PRG continues to innovate and add improved value to its range based on emerging trends and needs of the market.
PRG is truly committed to a tradition of trusted quality in its entire operation and commercial processes.
Anniversary launch
Given all these remarkable achievements worth celebrating, the company launched its 60th anniversary celebration in Accra on May 12, 2022.
There are a series of activities lined up to mark the 60th anniversary which is being held on the theme “Trusted quality for great health: 60 years of continuous impact.”
The activities, which will be climaxed with a grand durbar in August this year, include donations to selected hospitals and medical institutions in May, an Accra roadshow to increase public awareness of health-related issues in August, Kumasi roadshow in October, and games in December this year.
Brighter future ahead
Addressing stakeholders at the launching ceremony, the Chief Executive Officer (CEO) of PRG (GIHOC), Mrs. Theresa Yamson, acknowledged the immense contributions of suppliers, the FDA, and other regulatory stakeholders as well as staff, both past and present, who worked hard and continued to work every day to deliver the utmost best to “take our company forward.”
“Today, as we commence with the launch of our activities to commemorate 60 years of continued impact, I am confident that the next 60 years will be even brighter and more successful for Phyto-Riker Pharmaceuticals so that generations to come will understand the depth of the vision started by our forefathers and value the economic and social development that comes with having a strong and vibrant pharmaceutical manufacturing sector here in Ghana,” she said.
Embrace disruptive innovations
The Board Chairman of PRG, Mr. Kwesi Atuah, stressed the need for the company to embrace technological innovations in order to allow the sail to a greater height.
“My challenge to you is that let us embrace innovation and disruption; let us find out what we can do and go ahead to do it to keep Phyto-Riker going,” he said. [...]
Read more...
Big salute to all those who struggled for the freedom of Ghana on the occasion of Ghana’s Independence Day. Being the premier pharmaceutical company in Ghana, celebrating this day is always a joyous one to behold and reminds us of our heritage. [...]
Read more...